Ammonia
Imagine a bustling society of tiny, determined beings—the autotrophic bacteria—living in a vibrant, unseen world. These microscopic citizens thrive in environments rich with ammonia, a substance that many consider waste, yet for them, it’s a treasure trove of energy.
In this miniature realm, the autotrophic bacteria are akin to resourceful warriors, battling against the harsh conditions of their surroundings. They form intricate communities, each member armed with unique abilities to harness energy from ammonia. As they oxidise this nitrogen-rich compound, they engage in a relentless struggle for survival, converting ammonia into energy through a sophisticated process akin to a bustling factory.
Picture them as skilled artisans, meticulously crafting energy from the raw materials at their disposal. They employ intricate biochemical pathways, transforming ammonia into essential nutrients that sustain their lives. This transformation is not without its challenges; they must navigate a landscape filled with competition and changing conditions, constantly adapting their strategies to ensure their survival.
As they work together, these bacteria form alliances, sharing resources and knowledge. Some specialize in breaking down ammonia quickly, while others focus on storing energy for leaner times. In this cooperative spirit, they create a resilient network, much like a community of people pooling their resources to weather a storm.
Amidst this struggle, they also face predators—other microorganisms that threaten their existence. Yet, through their ingenuity and teamwork, the autotrophic bacteria find ways to outsmart these challenges. They develop protective barriers and employ defensive tactics, ensuring that their society continues to flourish in the face of adversity.
In their quest for survival, these tiny warriors exemplify determination and resilience. They remind us that even in the smallest corners of the world, life finds a way to adapt, thrive, and create energy from the most unexpected sources. As they continue their fight, the autotrophic bacteria not only sustain themselves but also contribute to the greater ecosystem, playing a vital role in the nutrient cycles that support life on Earth.
In the intricate tapestry of microbial life, heterotrophic bacteria play a crucial role, much like scavengers in a vibrant ecosystem. These bacteria thrive by consuming organic matter, breaking down dead and decaying material to extract the nutrients they need to survive. Their activities have profound implications for the autotrophic community that relies on the very processes these heterotrophs undertake.
Imagine a bustling environment where life and death coexist in a delicate balance. As organic matter—whether it be fallen leaves, dead animals, or even waste—accumulates, heterotrophic bacteria spring into action. They are the cleanup crew, tirelessly consuming this biomass and converting it into simpler compounds. In doing so, they release nutrients into the surrounding environment, such as carbon, nitrogen, and phosphorus, which are essential for the growth and survival of autotrophic bacteria.
For the autotrophic community, which thrives on inorganic substances, this nutrient release is akin to a bountiful harvest. The autotrophic bacteria, who depend on processes like photosynthesis or chemosynthesis, benefit from the organic compounds broken down by their heterotrophic counterparts. The nutrients made accessible by heterotrophs fuel the autotrophs' energy-producing pathways, allowing them to flourish and maintain their vital roles in the ecosystem.
However, this relationship is not without its complexities. As heterotrophic bacteria consume organic matter, they can also outcompete autotrophic bacteria for limited resources, especially in nutrient-dense environments. In this struggle for survival, autotrophic bacteria must adapt, finding ways to optimize their nutrient uptake and energy production to coexist with their heterotrophic neighbors.
Furthermore, the activities of heterotrophic bacteria can influence the overall health of the ecosystem. In some cases, an overabundance of heterotrophs can lead to nutrient depletion or even harmful algal blooms, which may disrupt the balance of the community. This shift can create challenges for autotrophic bacteria, as changes in nutrient availability can directly impact their productivity and survival.
In essence, heterotrophic bacteria serve as both allies and competitors in the microbial world. Their ability to decompose organic matter and recycle nutrients is vital for sustaining the autotrophic community, yet their unchecked proliferation can pose challenges. Together, these two groups of bacteria embody the interconnectedness of life, demonstrating how the cycles of consumption and decomposition drive the health of ecosystems and the survival of diverse microbial communities.
In the context of a nitrifying process in wastewater treatment, both autotrophic and heterotrophic bacteria have distinct roles, and their effects can vary:
### Autotrophic Bacteria
- **Good for Nitrification**: Autotrophic bacteria, particularly nitrifying bacteria like *Nitrosomonas* (which oxidizes ammonia to nitrite) and *Nitrobacter* (which oxidizes nitrite to nitrate), are essential for the nitrification process. They derive energy from inorganic substances, specifically ammonia and nitrite, making them crucial for converting harmful nitrogen compounds into less harmful forms.
- **Synergistic Relationship**: Their presence is vital for maintaining the nitrogen cycle in the treatment system, and they often benefit from the nutrients that may be released by heterotrophic bacteria.
### Heterotrophic Bacteria
- **Role in Organic Matter Breakdown**: Heterotrophic bacteria consume organic matter, which can help reduce biochemical oxygen demand (BOD) in wastewater. They can release nutrients that support the growth of autotrophic bacteria.
- **Potential Issues**: However, if heterotrophic bacteria dominate, they can lead to competition for nutrients and oxygen, which may hinder the performance of autotrophic bacteria. Excessive growth of heterotrophs can also contribute to bulking, potentially leading to poor settling of sludge.
### Filamentous Bacteria
- **Filamentous Bacteria**: These can be either autotrophic or heterotrophic, but they are often associated with bulking issues in activated sludge systems. If filamentous bacteria proliferate excessively, they can disrupt the settling process, regardless of whether they are autotrophic or heterotrophic.
### Conclusion
- **Autotrophic Bacteria**: Generally beneficial for nitrification.
- **Heterotrophic Bacteria**: Can be beneficial for organic matter breakdown but may pose challenges if they outcompete autotrophs or contribute to bulking.
- **Filamentous Bacteria**: Their role can be complex; they can be both beneficial and detrimental, depending on their population dynamics and the overall balance in the microbial community.
Optimizing the conditions in the nitrifying process tank is essential to maintain a healthy balance between these bacterial groups to ensure efficient wastewater treatment.
Filamentous bacteria can play a significant role in the nitrifying process within an effluent treatment plant, but their necessity depends on the specific operational goals and conditions of the treatment system. Here are some key points to consider:
### Role of Filamentous Bacteria
1. **Stabilization of Sludge**: Filamentous bacteria can help stabilize the sludge in a nitrifying process tank. They contribute to the formation of a healthy floc structure, which is essential for effective sedimentation and separation of solids from the liquid effluent.
2. **Enhancing Microbial Community**: They often form part of the diverse microbial community that includes nitrifying bacteria (such as *Nitrosomonas* and *Nitrobacter*). A diverse community can improve the overall efficiency of the nitrification process.
3. **Nutrient Cycling**: Filamentous bacteria can assist in nutrient cycling by breaking down organic matter and releasing nutrients that nitrifying bacteria can utilize.
### Potential Issues
1. **Bulking Problems**: In some cases, excessive growth of filamentous bacteria can lead to bulking, which impairs the settling of sludge and can disrupt the treatment process. This can result in an increase in suspended solids in the effluent.
2. **Control Measures**: If filamentous bacteria become dominant, it may be necessary to implement control measures, such as optimizing aeration, adjusting nutrient levels, or employing specific chemical treatments to manage their growth.
### Conclusion
While filamentous bacteria can be beneficial in a nitrifying process tank by aiding in sludge stabilization and contributing to the microbial community, their presence must be carefully monitored. The goal is to maintain a balanced microbial ecosystem that maximizes nitrification efficiency while preventing issues like bulking. Adjustments in operational parameters may be needed based on the specific conditions of the treatment plant.
Nitrifying bacteria are a group of bacteria that are capable of utilizing ammonia and nitrite as energy sources that allow them to synthesize carbohydrates from carbon dioxide. They are highly significant to the global nitrogen cycle and to the existence of plants. They are autotrophs (they eat themselves) and they make ‘food ‘(carbohydrates) by utilizing energy from chemicals rather than from light as photosynthetic autotrophs do.
Taxonomy and Phylogeny
Nitrifying bacteria are not unified by phylogeny and the taxonomic entity ‘nitrifying bacteria’ is totally artificial. The group includes bacteria from several unrelated groups and also includes some archaea (i.e. organisms that are not bacteria). The group includes organisms that: (1) convert ammonia to nitrite, (2) convert nitrite to nitrate and (3) recently discovered organisms that can do both reactions, converting ammonia to nitrate.
Structure
Consistent with their phylogenetic diversity, these bacteria exist in a variety of forms: rods, spheres and spirals. Many have extensive internal membrane systems that probably are significant to the biochemistry they accomplish.
Matter and Energy
Most organisms obtain energy through cellular respiration, a series of biochemical reactions that remove hydrogens from carbohydrates (oxidizing them) and transfer them to oxygen, forming water. In the process, the energy from carbohydrates is transferred to ATP. One can think of ammonia as ‘food’, comparable to carbohydrates in being a chemical that can be oxidized in a process that allows its energy to be ‘captured’. Some nitrifying bacteria are ‘ammonia oxidizing bacteria’ and they obtain energy as the ammonia is oxidized to nitrite (NO2–).
In a similar process, the nitrite (NO2–) formed by ammonia oxidizing bacteria can be further oxidized by ‘nitrite oxidizing bacteria’, converting the nitrite (NO2–) to nitrate (NO3–).
Each of these groups have members that can do more than just acquire energy in the form of ATP as they carry out these oxidations. Both These reactions can also be part of a process that allows carbohydrates to be formed from carbon dioxide. Recall that this happens in the Calvin cycle of photosynthesis where NADPH (a source of electrons, i.e. reducing power) and ATP are inputs in a process that reduces carbon dioxide, forming carbohydrates. In nitrifying bacteria the net effect of the process is a transfer of electrons from either ammonia or nitrite to CO2:
ammonia + CO2 —-> NO2– + (CH2O)n (ammonia oxidizers)
NO2– + CO2 —-> NO3– + (CH2O)n (nitrite oxidizers)
ammonia + CO2 —-> NO3– + (CH2O)n (comammox)
Organisms that carry out these reactions are considered autotrophs because they eat self-made food and they could be called ‘chemoenergetic autotrophs’ because they utilize chemical energy to make their on food (similarly plants could be considered ‘photoenergetic autotrophs’). Other organisms in these groups do not make their own food but simply use ammonia and nitrite as a food source to get energy. They need to acquire reduced carbon to satisfy their material carbon needs. They could be considered chemoenergetic heterotrophs.
Interactions
Nitrifying bacteria are extremely important to all vascular plants by providing the preferred form of nitrogen (nitrate) to the soil and allowing nitrogen to cycle rapidly from plants to heterotrophs to nitrifying bacteria and back to plants (Chapter 22).
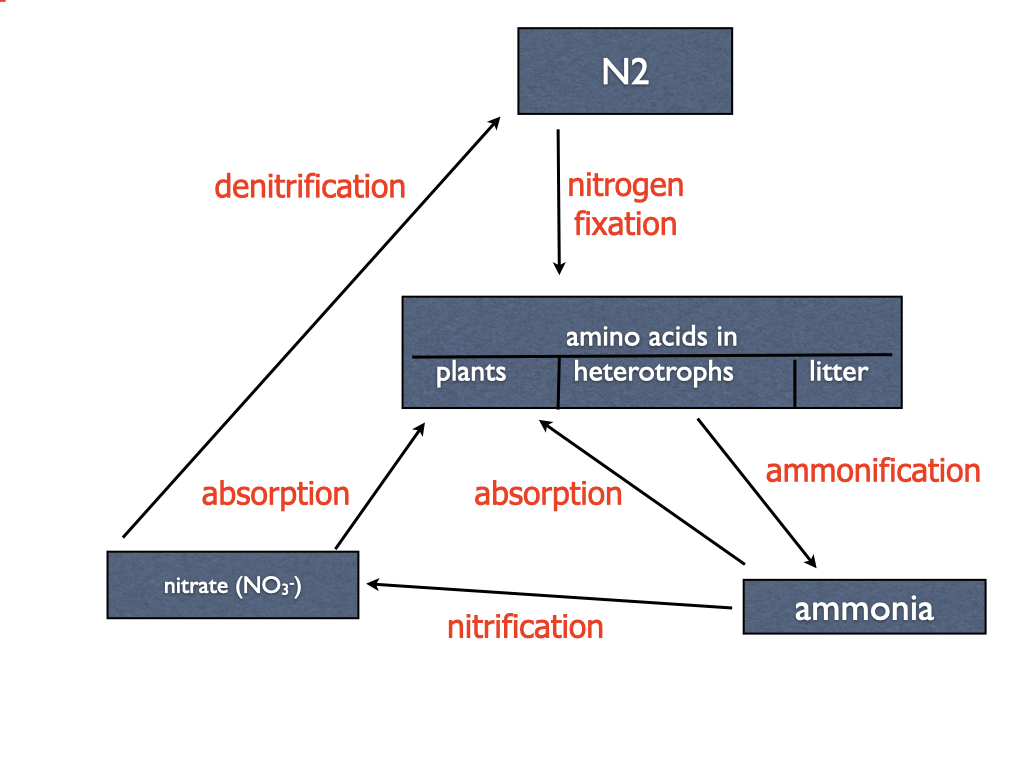
Nitrifying bacteria conserve nitrogen in the soil because ammonia is volatile and escapes to the atmosphere, although in acid soils it gets protonated to ammonium (NH4+) that is not volatile.
Most biological nitrogen fixation occurs by the activity of molybdenum (Mo)-nitrogenase, found in a wide variety of bacteria and some Archaea. Mo-nitrogenase is a complex two-component enzyme that has multiple metal-containing prosthetic groups
- Ammonia (NH3) or ammonium (NH4+) is oxidized to nitrite (NO2-) by ammonia-oxidizing bacteria (AOB).
- Nitrite (NO2-) is oxidized to nitrate (NO3-) by nitrite-oxidizing bacteria (NOB).
Ammonia monooxygenase (AMO) is an enzyme that contains copper, iron, and zinc, and is made up of subunits called AmoA, AmoB, and AmoC. AMO is responsible for the first step in the oxidation of ammonia to hydroxylamine.
- Copper: Up to 20 copper ions per heterotrimer, which may be involved in electron transfer or activating molecular oxygen
- Iron: Possibly nonheme iron
- Zinc: 0.5 to 2.6 mol per mol AMO
- AmoA: The alpha-subunit, with a molecular mass of around 27 kDa
- AmoB: The beta-subunit, with a molecular mass of around 42 kDa
- AmoC: A subunit of AMO
- AMO may be located in the membrane or in the cytoplasm of bacteria
- AMO may have a molecular mass of around 283 kDa
- AMO may have a sub-unit structure of alpha(3)beta(3)gamma(3)
- AMO catalyzes the reaction of ammonia, AH₂, and O₂ to produce NH₂OH, A, and H₂O
- AMO is the first enzyme in the oxidation of ammonia
- Copper in microbial pathogenesis: Copper is an essential cofactor for some microbial enzymes, and it can also restrict pathogen growth.
- Copper in filamentous fungi: Filamentous fungi have been isolated that can remove copper from the environment.
- Copper alloys: Copper alloys like brass and bronze have antimicrobial properties.
- Zinc in cyanobacteria: Cyanobacteria have zinc in carboxysomal carbonic anhydrase.
- Zinc in metallo-β-lactamases: Metallo-β-lactamases are enzymes that use zinc ions to inactivate β-lactam antibiotics.
- Zinc in Streptomyces zinciresistens: Streptomyces zinciresistens is a filamentous bacterium that is resistant to zinc.
- Biosorption: Filamentous fungi can be used to remove heavy metals from the environment.
- Copper homeostasis: Copper homeostasis systems are important for the virulence of some pathogenic fungI
Comments
Post a Comment